Auto logout in seconds.
Continue LogoutScientists are working on a new generation of allergy treatments that would stop allergies "in their tracks" as opposed to just reducing their symptoms—although some experts are skeptical of the approach, Meghana Keshavan writes for STAT News.
The potential market for these treatments is "huge," according to Keshavan. Fifty million people in the United States suffer from allergies. According to the 2014 National Health Interview Survey, up to 10 percent of children get hay fever, nearly 12 percent experience skin allergies, and 5 percent have food allergies.
Treatments in the works
One potential treatment that might be closest to market is Aimmune's peanut desensitization pill, Keshavan reports. The Bay Area startup is developing the product in part with $145 million in support from Nestle.
The pill gradually "weans patients away from allergy," Keshavan writes.
To make the medication, Aimmune identified the peanut proteins that cause allergic reactions and put those proteins into pill capsules. Patients initially take a half a milligram of peanut protein, then incrementally up their dosage over roughly six months, to a point when they consume the equivalent of one peanut.
Aimmune's peanut powder is in Phase 3 clinical trials.
Meanwhile, researchers at Stanford University are testing whether omalizumab—a biologic approved by FDA in 2003 to treat a type of asthma typically brought upon by allergens—can help patients who have food allergies.
Genentech markets omalizumab under the brand name Xolair. The drug is an engineered antibody that stops immunoglobulin E, the antibody that causes an allergic reaction, from activating.
Stanford researchers have found that the drug speeds up the allergy desensitization process, which often takes years to treat a single allergy, according to Toshi Kawakami, a researcher at the La Jolla Institute for Allergy and Immunology.
Andrew Long, the lead investigational drug pharmacist at Stanford's Sean N. Parker Center for Allergy & Asthma Research, told STAT News that the lengthy timeline can mean desensitization isn't a viable option for patients with more than one allergy, who make up about 70 percent of patients with food allergies.
But Stanford researchers have found that combining omalizumab with desensitization therapy can treat up to five food allergens at a time, speeding up the process.
Meanwhile, Japan's Astellas Pharma is developing a vaccine that could be used to combat cedar pollen allergies.
The underlying research comes from Immunomic Therapeutics and Johns Hopkins University. The research involves attaching a piece of DNA to a vaccine that can be embedded within the cell. The DNA then creates an immune response and leaves an "immunological memory"—meaning the immune system will respond quicker to future exposure to the allergen.
The goal is to develop resistance to the allergen without exposing the patient to that substance, Keshavan reports. Should the vaccine work, it would "be fairly easy" to insert DNA for other allergens into the template, Keshavan writes.
Long noted that, compared with other methods, this approach "hypothetically decreases the risk for adverse events" because "patients aren't exposed to the circulating antigen."
Skepticism
Nonetheless, there's doubt about whether these new approaches to treating allergies will bear fruit, Keshavan reports.
For instance, Circassia Pharmaceuticals' immunotherapy drug to forestall cat allergies in a Phase 3 study was found to be no more effective than a placebo.
In addition, current allergy treatment medications, such as Benadryl, Claritin, and epinephrine, effectively control symptoms for most people, which can discourage innovation.
There's also the issue of liability concerns. Keshavan writes, "It's a risky proposition to give patients the foods they're deathly allergic to."
Wayne Shreffler, a researcher at Massachusetts General Hospital who is working on the Broad Institute's Food Allergy Science Initiative, said, "There's really no doubt that there have been cold feet in the industry about this issue" (Keshavan, STAT News, 11/29).
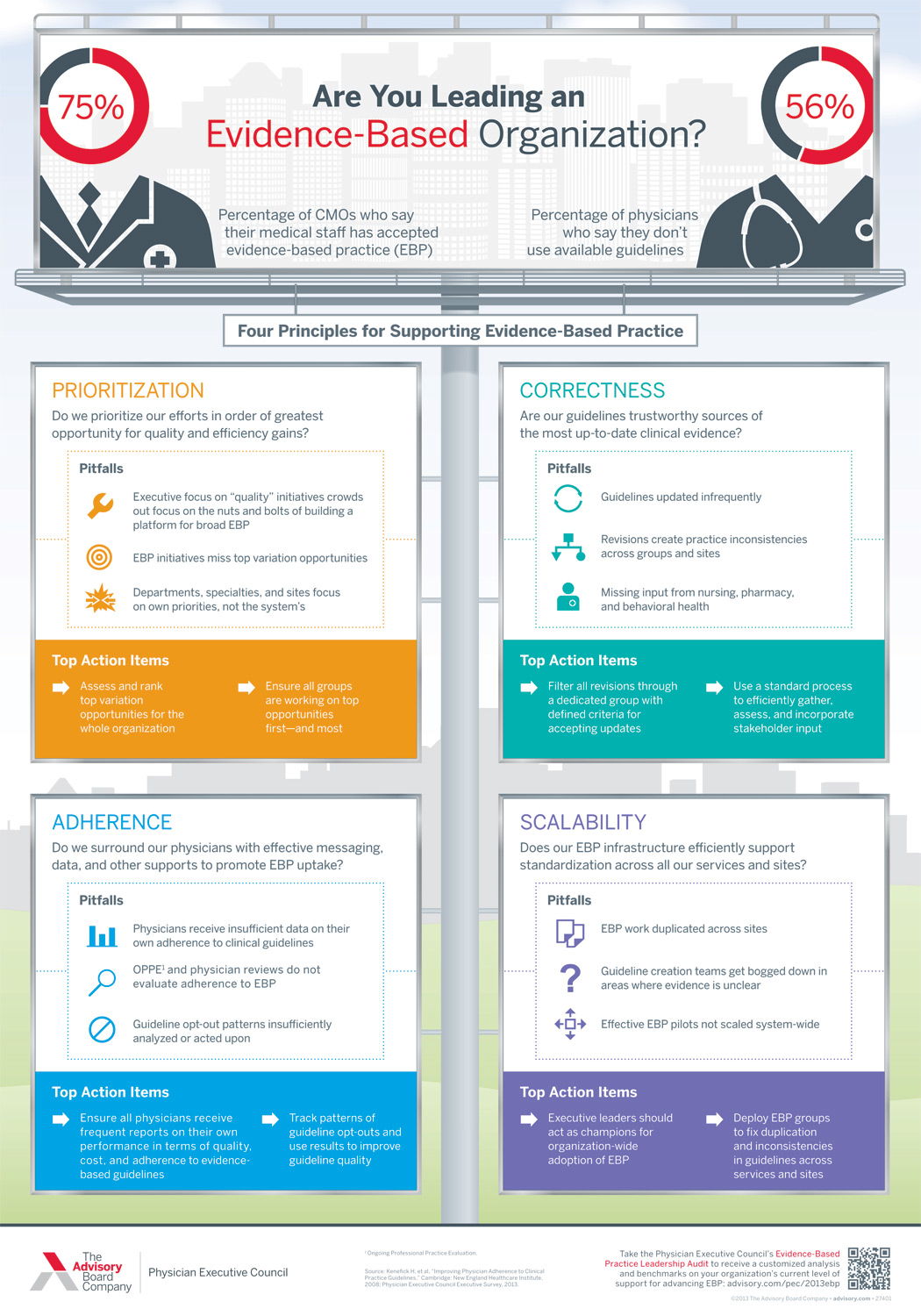
Are you leading an evidence-based organization?
Despite the shift toward broad acceptance of evidence-based practice (EBP) among medical staff, over half of physicians report not actually using guidelines day-to-day when they are available. As a result, organizations continue to see tremendous variation in clinical practice—as well as in costs and outcomes.
Our infographic outlines four principles you can use to support EBP at your organization, along with action steps to implement each one and pitfalls to avoid along the way.
Don't miss out on the latest Advisory Board insights
Create your free account to access 1 resource, including the latest research and webinars.
Want access without creating an account?
You have 1 free members-only resource remaining this month.
1 free members-only resources remaining
1 free members-only resources remaining
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
This content is available through your Curated Research partnership with Advisory Board. Click on ‘view this resource’ to read the full piece
Email ask@advisory.com to learn more
Click on ‘Become a Member’ to learn about the benefits of a Full-Access partnership with Advisory Board
Never miss out on the latest innovative health care content tailored to you.
Benefits Include:
This is for members only. Learn more.
Click on ‘Become a Member’ to learn about the benefits of a Full-Access partnership with Advisory Board
Never miss out on the latest innovative health care content tailored to you.