Auto logout in seconds.
Continue LogoutPrecision medicine brings the promise of providing more targeted treatment to cancer patients—as a result, it has generated a lot of excitement among cancer providers, administrators, and patients. But as the field rapidly evolves, cancer programs are challenged to stay on top of advances, make informed investments, and help providers put precision medicine into practice.
Read on to learn what over 170 programs that participated in our Trending Now in Cancer Care survey told us about how they offer molecular testing and immunotherapy to their patients.
Majority of cancer programs offer small and large panel molecular testing
Almost all respondents reported that their organization sends out biopsies for molecular testing. Predictive assays (e.g., OncotypeDX) and single gene testing (e.g., KRAS, EGFR, HER2) were by far the most common, with 92% of respondents reporting that their organization sends out biopsies for these tests. 72% of respondents send out biopsies for small panel tests and 56% perform biopsies for large panel tests. Whole exome/genome sequencing was the least common molecular test, but still 31% of respondents indicate that their organization will provide samples for these tests.
Which of the following types of molecular testing does your program send biopsy samples for?1
Percentage of respondents, 2017
n=171

1) Respondents were asked to select all that apply
Learn about how to develop a molecular testing strategy
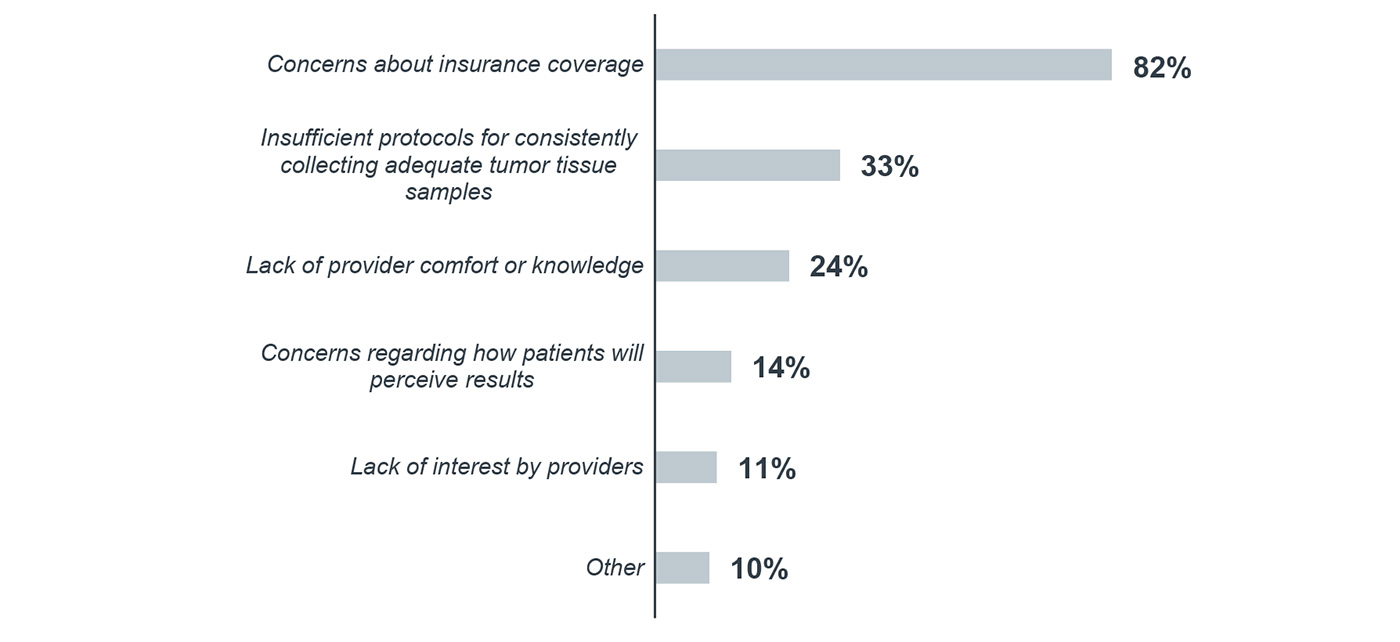
Concerns about insurance coverage identified as most common barrier for molecular testing
Most cancer programs (82%) cited concerns about insurance coverage as the greatest barrier to expanding molecular testing at their organization. Other common concerns include insufficient protocols for consistently collecting adequate tumor tissue samples (33%) and lack of provider comfort or knowledge (24%).
One strategy to enhance the quality of biopsies at your organization is to improve coordination with interventional radiologists. Our research briefing, Innovations in Interventional Oncology, includes three models to improve the quality of biopsies at your organization.
What barriers prevent additional molecular testing at your program1
Percentage of respondents, 2017
n=153
1) Respondents were asked to select all that apply
Many programs missing out on benefits of molecular tumor boards
We also asked respondents about oncologist participation in molecular tumor boards. Molecular tumor boards provide ongoing education and can help providers determine the best treatment plan for a patient based on their molecular profile by consulting with other experts and peers. However, only 27% of survey respondents indicated that their oncologists participated, 65% said their oncologists do not participate, and 9% were unsure.
Do your oncologists participate in a molecular tumor board?
Percentage of respondents, 2017
n=175
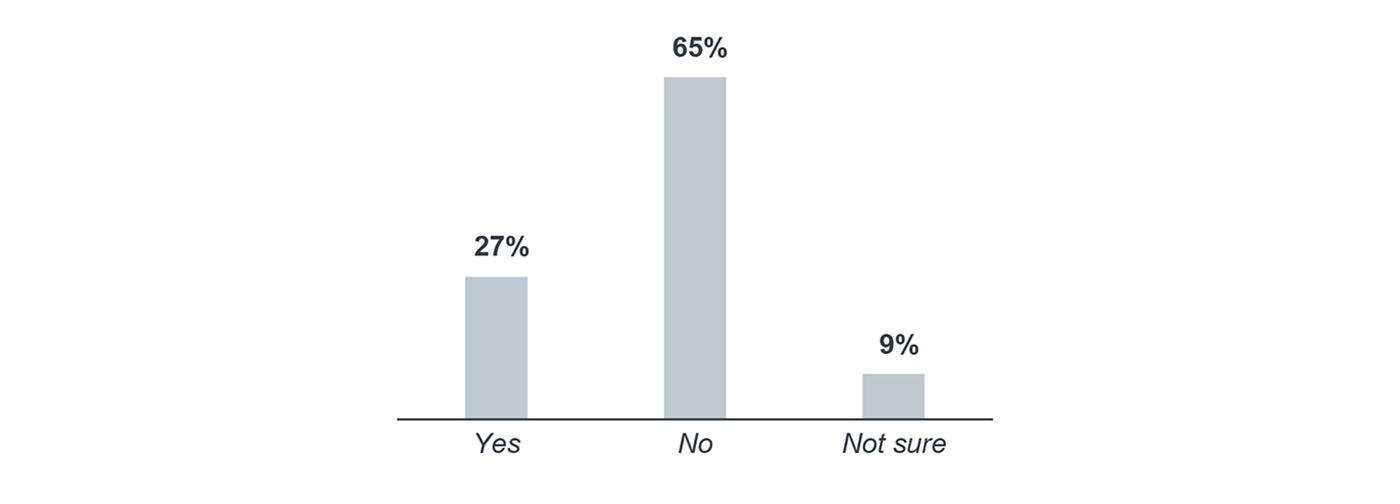
Even if your organization does not offer molecular tumor boards, there are a variety of ways for oncologists to participate. If your organization outsources molecular testing, choose a lab that provides access to molecular tumor boards alongside test results. Some larger institutions, such as Intermountain, also open molecular tumor board participation to external providers.
Use of immunotherapy grows, but many providers uncomfortable managing the side effects
An overwhelming 96% of respondents reported that providers in their program prescribe immunotherapy agents (e.g., nivolumab, pembrolizumab). The majority of programs (78%) often or always test for biomarker expression (e.g., PD-1,PD-L1 inhibitors) prior to treatment.
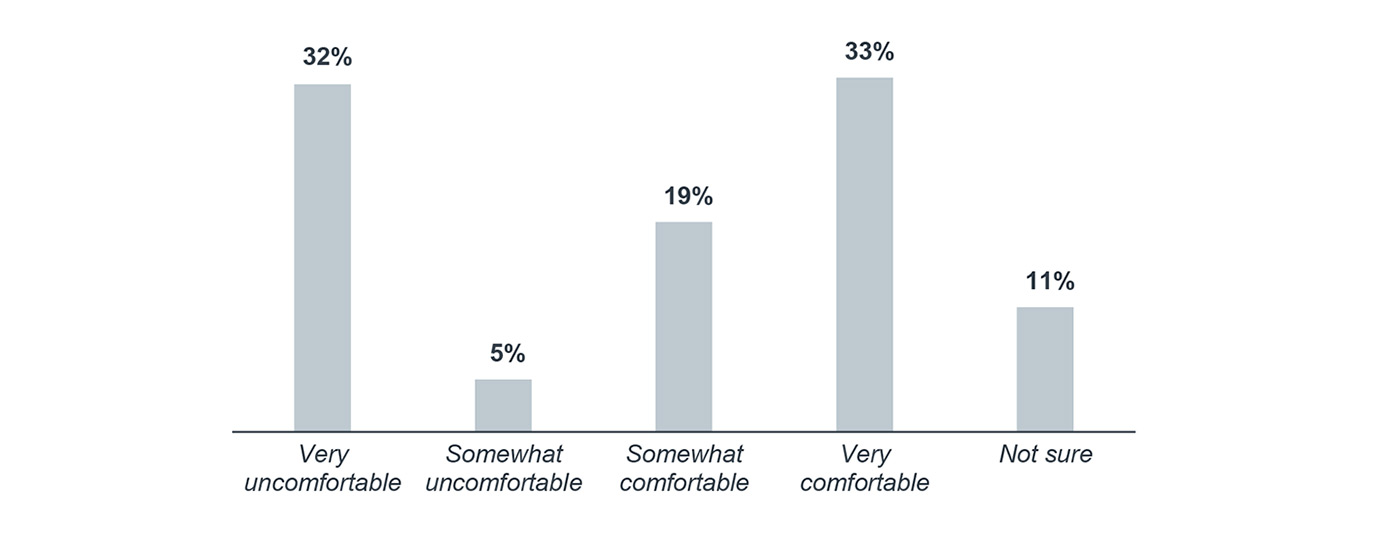
Although immunotherapy agents are commonly prescribed, providers are split on their comfort level managing associated adverse events and side effects. One-third of respondents reported that providers at their organization feel very comfortable managing immunotherapy-related side effects, but another third of respondents said their providers are very uncomfortable managing these side effects.
How comfortable are your providers with managing immune-related adverse events and side effects?1
Percentage of respondents, 2017
n=166
1) Respondents were only asked this question if they indicated that their providers prescribe immunotherapy agents if appropriate
Since the majority of organizations are providing immunotherapy agents, it is essential that all providers are comfortable managing any related side effects or adverse events. The American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) just released clinical guidance for the management of side effects caused by immunotherapy. In addition, molecular tumor board participation is another way for oncologists to confer about potential side effects of their patients’ treatment regimens and learn best practices for how to effectively prevent and manage these events from their peers.
For more information on precision medicine, see our research briefing, Innovations in Medical Oncology.
Strategies for success in the era of precision medicine
Join us for a webconference on Tuesday, March 20 at 3 p.m. ET to learn about new and emerging innovations in medical oncology, including targeted therapies, immunotherapy, and next-generation sequencing, and how your program can prepare for these exciting discoveries.
Don't miss out on the latest Advisory Board insights
Create your free account to access 1 resource, including the latest research and webinars.
Want access without creating an account?
You have 1 free members-only resource remaining this month.
1 free members-only resources remaining
1 free members-only resources remaining
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
This content is available through your Curated Research partnership with Advisory Board. Click on ‘view this resource’ to read the full piece
Email ask@advisory.com to learn more
Click on ‘Become a Member’ to learn about the benefits of a Full-Access partnership with Advisory Board
Never miss out on the latest innovative health care content tailored to you.
Benefits Include:
This is for members only. Learn more.
Click on ‘Become a Member’ to learn about the benefits of a Full-Access partnership with Advisory Board
Never miss out on the latest innovative health care content tailored to you.
