Auto logout in seconds.
Continue LogoutWhat is oncology biomarker testing?
Oncology biomarker testing (also known as mutation, genomic, or molecular testing) uses laboratory tests, including companion diagnostics, to help the health care teams gather as much information as possible about a patient’s cancer, ideally before treatment begins, and uncover whether the patient has an actionable biomarker. A companion diagnostic (CDx) is typically an in vitro diagnostic that detects a perfective biomarker to determine whether the patient will respond to a specific treatment.
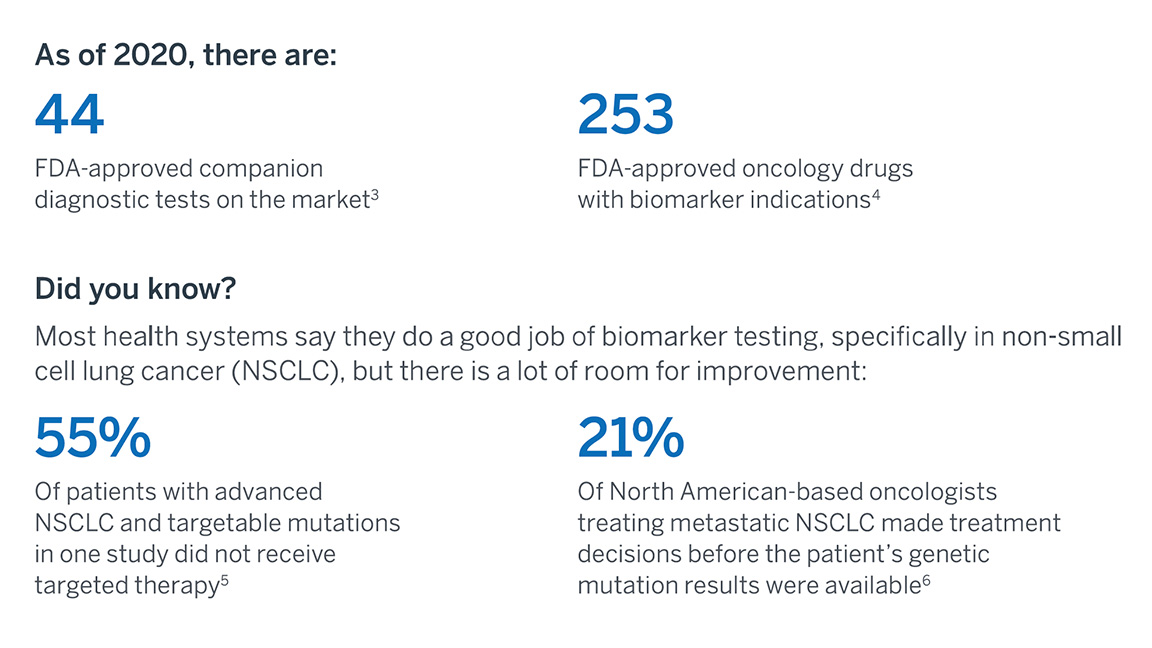
Benefits of Oncology biomarker testing
Health systems
Advisory Board’s research has found that oncology biomarker testing aligns with many of a health system’s broader strategic goals. In the future, health systems will increasingly use oncology biomarker testing to deliver personalized care to patients and advance their precision medicine initiatives.
Biomarker testing can also help organizations drive towards value-based care by improving the overall value of care across the entire patient journey—from diagnosis to treatment selection and outcomes.
Additionally, organizations can use biomarker testing to elevate their oncology department. Advisory Board found that best-in-class organizations utilize biomarker testing to help improve outcomes and strengthen the quality of the oncology care they provide.
Providers
Providers can use oncology biomarker testing to help make more informed care and treatment decisions based on a patient’s genetic makeup.
Notably, biomarker testing can help providers get patients on the right treatment, at the right time. In fact, 37.6% of cancer patients in one study of 5954 patients with refractory malignancies had therapeutically actionable genetic alterations, meaning they could be matched to existing drugs, across many different cancer types. Providers can also use biomarker testing to indicate a patient’s increased risk or susceptibility to a given disease, predict the natural history of disease without further intervention, or predict an outcome following an intervention.
Patients
Oncology biomarker testing may help improve patients’ treatment success and outcomes. It also allows patients to avoid trial-and-error and more quickly receive the appropriate treatments for their genetic makeup.
Your role in advancing biomarker testing
Recommendations from Advisory Board’s research
- Assess how biomarker testing supports your strategic priorities.
Consider how biomarker testing can support your broader organizational goals—whether delivering high-value and appropriate care, advancing precision medicine, or others.
- Connect with your VP/service line leader to gauge current performance.
Evaluate your organization’s current biomarker testing program and processes. Discuss what works well and identify any challenges or barriers when it comes to biomarker testing. Use these discussions to recognize opportunities to standardize biomarker testing across your broader organization.
- Invest in necessary infrastructure (e.g., staff, EHR enhancements) to improve process and long-term results.
Leverage any internal resources (e.g., staff, EHR enhancements) to improve your biomarker testing process. Prioritize among long-lasting, sustainable investments to improve your biomarker testing process.
AstraZeneca focuses on the discovery, development and commercialisation of prescription medicines in Oncology and BioPharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology.
This brochure is sponsored by AstraZeneca for educational purposes only. The content, views, and opinions contained within the cheat sheet are copyrighted by Advisory Board and all rights are reserved. Advisory Board experts wrote the content, conducting the underlying research independently and objectively. Advisory Board does not endorse any company, organization, product or brand mentioned herein.

This brochure is sponsored by AstraZeneca. Advisory Board experts wrote the report, conducting the underlying research independently and objectively. AstraZeneca had the opportunity to review the brochure.
Don't miss out on the latest Advisory Board insights
Create your free account to access 1 resource, including the latest research and webinars.
Want access without creating an account?
You have 1 free members-only resource remaining this month.
1 free members-only resources remaining
1 free members-only resources remaining
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
This content is available through your Curated Research partnership with Advisory Board. Click on ‘view this resource’ to read the full piece
Email ask@advisory.com to learn more
Click on ‘Become a Member’ to learn about the benefits of a Full-Access partnership with Advisory Board
Never miss out on the latest innovative health care content tailored to you.
Benefits Include:
This is for members only. Learn more.
Click on ‘Become a Member’ to learn about the benefits of a Full-Access partnership with Advisory Board
Never miss out on the latest innovative health care content tailored to you.
