Auto logout in seconds.
Continue LogoutPatient centricity has been a longstanding aspiration for many health care organizations. But the push to understand patient-defined value, to enable shared decision-making, and to prepare for new regulatory requirements is creating more urgency today than ever before.
While progressive organizations are investing in capturing patient-reported outcomes (PROs), specific guidance for how PROs should inform treatment decisions remains sparse. At the same time, growth of digital technologies like wearables, remote monitoring, and EHR-based tools are creating new ways of capturing patient-focused data in real time.
So, how can providers meaningfully capture the patient voice through ePROs? How should PRO data inform treatment decisions and value analysis? What role should stakeholders across the health care ecosystem play in advancing the use of PROs?
Across the last four months, Advisory Board partnered with Pfizer Oncology to start to address these questions. Advisory Board spoke with 50+ leaders from across the healthcare industry to understand where the most compelling opportunities for ePROs exist—and where root cause barriers are preventing progress.
We also hosted a virtual workshop, sponsored by Pfizer, designed to unpack those questions, surface areas of shared aspiration, and identify opportunities for cross-industry collaboration. Pfizer participated in the workshop, along with a range of progressive organizations, like MD Anderson, Carevive, Tennessee Oncology, Patient Advocate Foundation, COTA, Memorial Sloan Kettering, UnitedHealthcare, One Oncology, Noona, Purchaser Business Group on Health, and many more.
Below are 5 key takeaways from the research and workshop discussion.
Across the last several years, there has been an uptick in efforts to meaningfully understand the patient voice—and to determine how and when patient voice should influence health care. While capturing patient voice is critical in all clinical contexts, it is an increasingly prominent goal in oncology, where patients are living longer and quality of life (QoL) is a crucial part of decision-making, given how severe the side effects of treatment can be.
Several industry segments—including providers, payers, life sciences firms, regulators, and technology companies—are investing in better understanding patient voice, but those efforts can look very different depending on the segment. For example, a life sciences firm may aim to incorporate the patient’s experience into treatment development decisions, to ensure new treatments address unmet needs in a meaningful way. A physician may want to better understand a patient’s preferences and engage them in shared decision-making. Payers may want to understand patients’ QoL outcomes and determine how to build better clinical pathways.
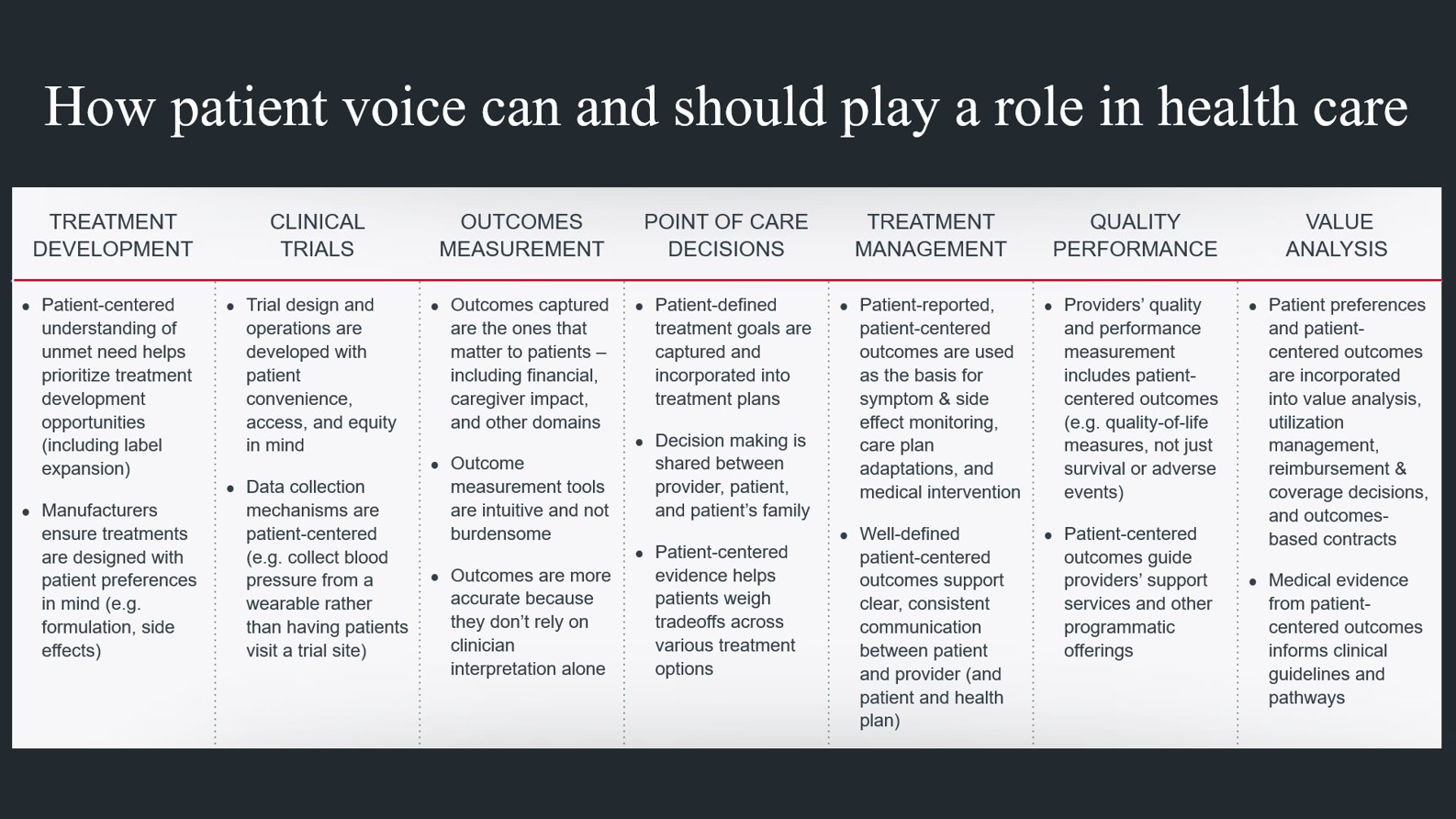
Don’t forget: Understanding the impact of social determinants of health on a patient’s preferences and needs is a critical piece of the patient voice. Many patients have access and affordability barriers that can impact outcomes, but those barriers are not always assessed in a systematic way.
As interest in amplifying patient voice grows, PROs are increasingly viewed as a valuable tool for capturing patient voice through data. PROs can help assess things like ongoing symptoms, side effects of treatment, and quality-of-life outcomes. In many cases, PROs capture information that only the patient can provide, like pain levels, nausea, fatigue, or depression—and quantifies the information so it can be measured over time.
Collecting PRO data in a consistent way can support a range of important goals. PROs can support care management efforts by tracking side effects and helping patients communicate about their needs. They can provide nuanced insight about the impact of treatments, beyond safety and efficacy. And they can even help prevent unnecessary utilization by flagging not just when medical intervention is necessary, but also when it’s not necessary.
Although PROs can be a valuable source of data, it’s important to remember that just because an outcome is reported by the patient doesn’t necessarily mean it is patient-centered. Outcomes are only patient-centered if they measure the concepts patients care about, if they are intuitive and not burdensome to report, and if they are used to inform care in a way that matters to patients—a bar that many PRO tools don’t currently meet.
"A lot of what we measure are things that aren’t actually important to the patient. The challenge is we don’t always have the tools to measure the things that do matter to patients."
- Patient Advocacy Leader
These nuances are frequently left out in industry conversations about patient voice. Three terms in particular—patient-reported outcomes, patient-centered outcomes, and patient-generated data—are frequently used interchangeably. In discussions about patient voice, especially involving stakeholders from different industry segments, it is critical to clarify language to avoid misinterpretation (and to find common ground).
Don’t forget: PROs are just one of many tools to help tap into the patient voice. Things like shared decision-making between physicians and patients, patient experience surveys, focus groups, and motivational interviewing can all help amplify the patient’s voice.
One of the most compelling use cases for PROs is in care delivery. (Historically, the use of PROs was largely limited to the clinical trial setting). During the course of routine care, providers can monitor patients using PROs and use them to inform treatment plan adjustments or assess the need for symptom management services.
Today, most of this monitoring happens reactively: patients report symptoms and side effects through validated surveys (referred to as patient-reported outcome measures, or PROMs) during scheduled visits with their doctor. Typically, patients are handed an iPad in the waiting room, and their reported data gets fed to the physician just before the visit takes place. In many cases, patients might report serious side effects like vomiting or extreme nausea. In this “reactive” model, the patient may have already discontinued their medication by the time the physician is aware of the problem.
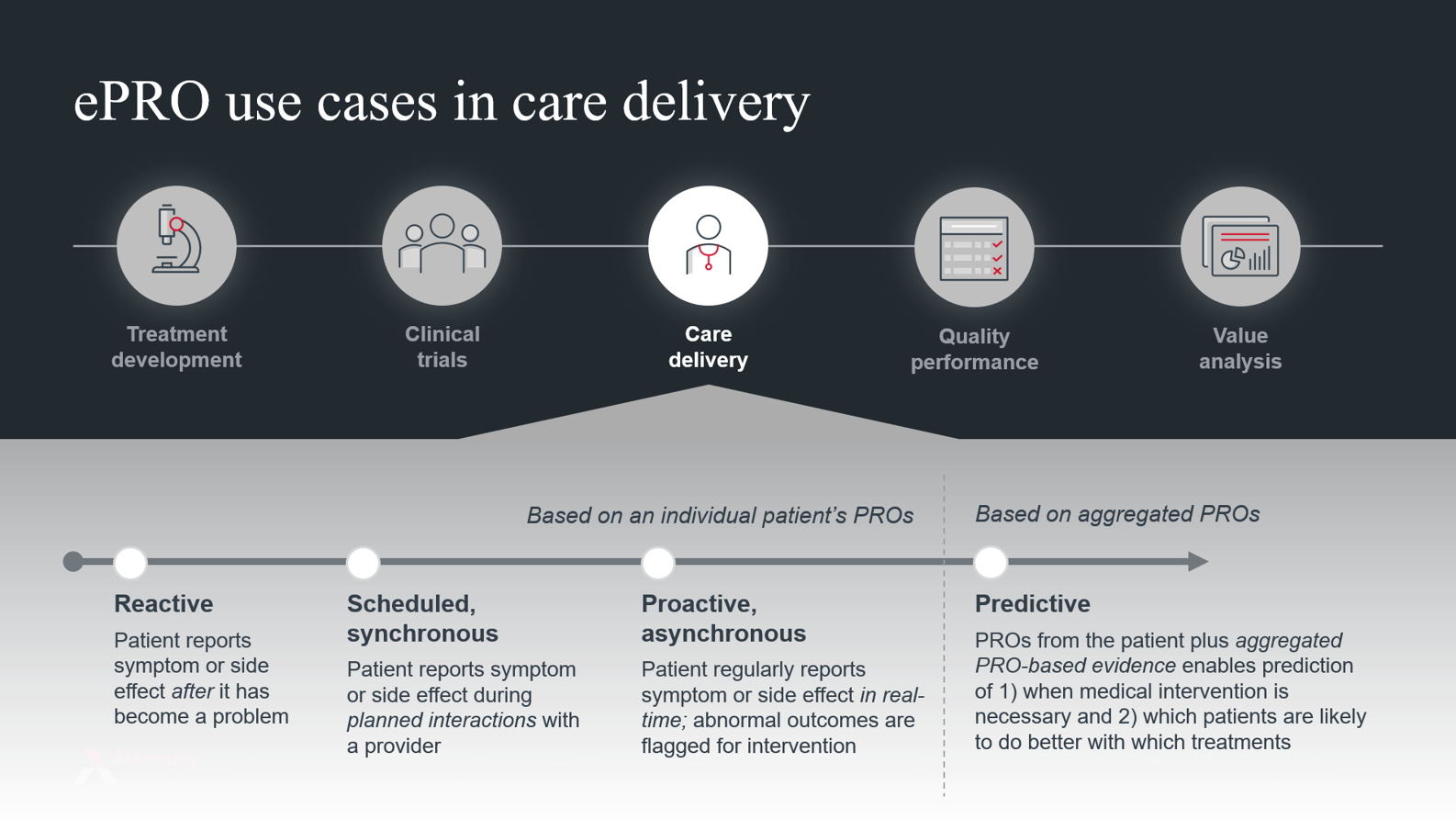
But as digital tools like smartphones, wearables, and patient portals become more widely adopted, there are new opportunities to collect PROs electronically, asynchronously, and in real-time. By capturing electronic PROs (ePROs) while patients are at home, outside of scheduled visits, providers are better equipped to provide proactive—as opposed to reactive—treatment management.
By collecting ePROs asynchronously, providers can leverage analytic tools to proactively flag outlier outcomes that require medical intervention. Conversely, if outcomes indicate the patient is doing well, it can reduce the need for in-person or resource-intensive touchpoints.
Ideally, ePRO-enabled treatment management can prevent unnecessary utilization, increase provider efficiency, and improve the patient experience. But today, a host of challenges around workflow, interoperability, and data standardization remain, which raise questions about how to scale this kind of program. And, even if ePROs enable more proactive “flagging” of patient issues, they do not necessarily help physicians determine the best course of action.
"One key barrier is providing clinicians with the ability to use the information from ePROs, especially when they haven’t been formally trained on it."
- Research Leader
Don’t forget: ePROs can’t be collected if they are not reported. The utility of ePROs requires patients to be actively involved in consistently and accurately sharing data. This requires patient education as well as feedback from physicians demonstrating that the patient’s reported data is being reviewed, understood, and acted upon.
"There are so many ePRO tools that collect data but don't provide anything back. Patients need to see value from what they’re sharing, in terms of how it impacts their support and navigation."
- Technology Executive
Don’t forget: ePROs are collected through devices such as smartphones, smartwatches, and laptops—technologies that much of the U.S. still does not have access to and/or cannot easily use. As ePROs become more integral to care delivery, health care leaders must account for this “digital divide” in their planning (and ensure products are designed for non-English speakers and those with audiovisual impairments).
Today, many provider organizations struggle to implement the tools and workflows required to collect ePROs in a robust, scaled way. ePROs are usually collected for an individual patient and used to inform that patient’s care. But ePRO data is rarely aggregated across large patient populations in part because there is huge variation in which PRO measures are used.
"I think the growing challenge is balancing the disease-specific and general measures being assessed. We’re talking about oncology now, but you could have parallel conversations with cardiologists, with rheumatologists…How can we make this work for multi-morbid patients?"
- Physician Executive
As ePROs become more standardized and adopted, organizations will start to generate large data sets that can be de-identified and used for research. In particular, the data can be used to gain a more nuanced understanding of the impact of treatments beyond safety and efficacy. For example, aggregated ePRO data can help physicians better understand not just overall clinical efficacy (e.g. 5-year survival) but also the likelihood and severity of side effects (e.g. pain, memory issues).
This more nuanced understanding of treatment impact will ultimately help patients and physicians make personalized, shared decisions. In particular, it can help patients weigh tradeoffs among treatment options. Many cancer patients have to make difficult tradeoffs between extending life and ensuring quality of life, but quality of life can mean different things to different people. For some, it is about being able to play with their grandchildren for as long as possible. For others, it is about preserving fertility. PROs can provide insight into many of these quality-of-life tradeoffs.
Enabling personalized, shared decision-making through ePROs has myriad benefits for stakeholders across the industry (not to mention patients). It can lead to improved outcomes, reductions in unnecessarily utilization, and even improvements in adherence: the more closely a treatment choice aligns to a patient’s individual value framework, the more likely they are to adhere to prescribed regimens.
Ultimately, generating this evidence requires cross-stakeholder partnerships. Medical executives at life science companies are invested in understanding treatment impact beyond safety and efficacy, but need PRO data from providers or technology vendors to do so. Meanwhile, providers want to advance evidence-based medicine programs but may struggle to finance ePRO programs on their own, making ePROs an area ripe for partnership.
Don’t forget: Medical data is only useful in shared decision-making if it helps patients weigh tradeoffs they care about. As life science firms and providers collect more ePRO data, it’s critical to ensure the data is rooted in endpoints that are patient-centered and can impact shared decision-making.
"We need to measure what is most important to the patient so we can have a feedback loop – we measure what’s important to patients so we can feed information back to them that’s relevant."
- Patient Advocacy Leader
PROs are not new. In fact, researchers and clinical trial leaders have been using PROs for at least three decades. But a set of longstanding barriers have prevented PROs from being widely adopted in care delivery, including things like workflow impact, a lack of physician buy-in, a misperception that PROs are “squishy” and not “objective”, and frustration that payers do not reimburse for resource-intensive data collection.
However, across Advisory Board’s interviews and throughout the workshop, it became increasingly clear that these oft-cited barriers are actually symptoms of two very thorny, difficult root cause issues:
- Collecting and using ePROs to meaningfully inform patient care—in oncology or elsewhere—inherently requires multi-stakeholder investment. For example, to overcome workflow issues associated with data collection, providers need technology that not only integrates with the EHR but also fits in with their workflow. To appropriately act on the information provided through ePROs, physicians need the right clinical guidelines and evidence. For researchers or life sciences firms to generate that evidence requires providers to collect data consistently (i.e. using a consistent set of PROMs, on a regular basis, for all eligible patients). While each stakeholder group—especially providers, researchers, advocacy groups, technology vendors, and life science firms—has a role to play in advancing the use of ePROs, true progress cannot happen in silos.
- Stakeholders are hesitant to invest in robust ePRO programs without proof of ROI, but establishing ROI requires some level of investment. Many providers, for example, hesitate to invest the time and resources required to stand up an ePRO program without proof that it will inflect outcomes, save costs, and improve quality. Similarly, life science firms hesitate to invest in PRO-based evidence generation if it’s unclear how the evidence will inflect clinicians’ or payers’ decisions. But in order to establish ROI—in terms of cost savings, quality improvements, or decision impact—more stakeholders must begin to pilot ePRO programs and measure their impact.
Despite these barriers, there are three key trends that could increase demand for ePROs and thereby accelerate adoption. First, a shift to value-based care would create the financial incentives to embed ePROs into the patient journey. Since PROs have significant potential to improve quality, reduce adverse events, and flag when interventions are and aren’t necessary, they will become an integral part of how organizations provide higher-quality care at lower costs. Second, the broader adoption of remote patient monitoring (RPM) technology, driven in large part by Covid-19, will establish much of the technological infrastructure required to support ePRO programs. And third, as more care shifts to the home setting, there will be more urgency to build the infrastructure to support patients asynchronously, outside of the physician’s office.
Achieving a collective ambition for ePROs in cancer care can’t happen unless we plan for and address the adaptive challenges, not just the technical challenges, standing in our way. We need to adapt our culture, thoughts, and behaviors in order to make space for the myriad changes this type of work requires. Doing so likely means challenging our entrenched ideas about our identities, roles, and objectives as health care leaders. If providers, patients, payers, and other industry leaders are not bought in emotionally on the value of ePROs as an integral part of delivering good patient care, then efforts to expand adoption are bound to stall. To advance ePROs, health care leaders should:
- Expand the definition of ROI beyond clinical and financial measures. As an industry, we need to think more broadly about how we define ROI to include measures of impact such as improved communication, patient engagement, adherence, patient loyalty, or whether an individual’s treatment goals were met.
- Use storytelling and case studies to elevate moments of impact. Data can’t always win hearts and minds the way stories can. By sharing stories of organizations that implemented ePRO programs and meaningfully impacted patients as a result, we can start to build the culture and buy-in required for behavior change.
- Collaborate to build consensus. All stakeholders can benefit from advancing ePROs, but progress requires consensus and collaboration. A starting point is to come together to build consensus around which PROMs and which “ROI” metrics are most meaningful—to lay the groundwork for collaborating to enable ePRO data collection and utility in patient care.
- Abbey Kaler, MS, APRN, FNP-C, Nurse Practitioner, Advanced Breast Cancer (ABC) Program, MD Anderson Cancer Center
- Abi Baldwin Medsker, MSN, RN, OCN, Associate Director, Product Development & Business Delivery, Memorial Sloan Kettering Cancer Center
- Ammu Irivinti, Principal, ICAREdata Use Case Lead, CodeX HL7 FHIR Accelerator, MITRE Corp.
- Becky Borgert, PharmD, BCOP, Senior Director of Oncology Clinical Strategy and Innovation, Magellan Rx Management
- Brian Morrissey, MBA, Vice President, Oncology National Customer Group, Pfizer
- Cardinale Smith, MD, PhD, Director of Quality for Cancer Services, Mount Sinai Health System
- Christine Gilroy, MD, MS, Associate Chief Medical Officer, Bright Health
- Claire Snyder, PhD, MHS, Professor of Medicine, Oncology, and Health Policy & Management; Program Director, Building Lifestyle, Outcomes, and Care Services Research in Cancer (BLOCS), Johns Hopkins Schools of Medicine and Public Health
- Dawn Holcombe, FACMPE, MBA, ACHE, President, DGH Consulting; Director, NAMCP Medical Directors Institute Oncology Council; Executive Director, Connecticut Oncology Association; President, National Oncology State Network
- Eleanor Perfetto, PhD, MS, EVP for Strategic Initiatives. National Health Council
- Emma Hoo, Director, Value-Based Purchasing, Pacific Business Group on Health
- Heather Jim, PhD, Senior Member and Program Co-Leader, Moffitt Cancer Center
- Heidi McClelland, PharmD, BCACP, Director, Oncology Health Data Analytics Specialist, Pfizer
- Holger Keim, PhD, MBA, Senior Director, North America Medical Affairs, Oncology, Pfizer
- Jennifer Malin, MD, PhD, Chief Medical Officer, Oncology and Genetics, UnitedHealthcare
- Laurie Smith, MBA, MPH, Vice President, Marketing and Business Development, WiserCare
- Lee Schwartzberg, MD, FACP, Chief Medical Officer & Board Member, OneOncology
- Maddy Herzfeld, RN, BSN, OCN, Founder and Vice Chairman, Carevive
- Manav Sevak, Chief Executive Officer & Co-Founder, Memora Health
- Michael Graff, Co-founder and Chief Product Officer, Navigating Cancer
- Natalie Dickson, MD, President, Chief Medical Officer, Tennessee Oncology
- Rebekah Angove, PhD, Vice President, Patient Experience and Program Evaluation, Patient Advocate Foundation
- Toni Perry, RN, MSN, Global Head of Evidence and Value, Noona by Varian Medical Systems
- Ty Gluckman, MD, FACC, FAHA, Medical Director, Center for Cardiovascular Analytics, Research, and Data Science, Providence St. Joseph Health
- Viraj Narayanan, MBA, SVP and General Manager, Life Sciences Business, COTA
At Pfizer Oncology, we are committed to advancing medicines wherever we believe we can make a meaningful difference in the lives of people living with cancer. Today, we have an industry-leading portfolio of 24 approved innovative cancer medicines and biosimilars across more than 30 indications, including breast, genitourinary, colorectal, blood and lung cancers, as well as melanoma.
This research and associated workshop were sponsored by Pfizer for educational purposes only. The content, views, and opinions contained herein are copyrighted by Advisory Board and all rights are reserved. Advisory Board experts wrote the content, conducting the underlying research independently and objectively. Advisory Board does not endorse any company, organization, product or brand mentioned herein.

This article is sponsored by Pfizer. Advisory Board experts wrote the article, conducting the underlying research independently and objectively. Pfizer had the opportunity to review the article.
Learn moreDon't miss out on the latest Advisory Board insights
Create your free account to access 1 resource, including the latest research and webinars.
Want access without creating an account?
You have 1 free members-only resource remaining this month.
1 free members-only resources remaining
1 free members-only resources remaining
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
This content is available through your Curated Research partnership with Advisory Board. Click on ‘view this resource’ to read the full piece
Email ask@advisory.com to learn more
Click on ‘Become a Member’ to learn about the benefits of a Full-Access partnership with Advisory Board
Never miss out on the latest innovative health care content tailored to you.
Benefits Include:
This is for members only. Learn more.
Click on ‘Become a Member’ to learn about the benefits of a Full-Access partnership with Advisory Board
Never miss out on the latest innovative health care content tailored to you.
