Auto logout in seconds.
Continue LogoutNovo Nordisk last week announced plans to cut 9,000 jobs, roughly 11% of its workforce, as part of an organizational restructuring amid growing competition in the obesity drug market, in today's bite-sized hospital and health industry news from New Jersey and Maryland.
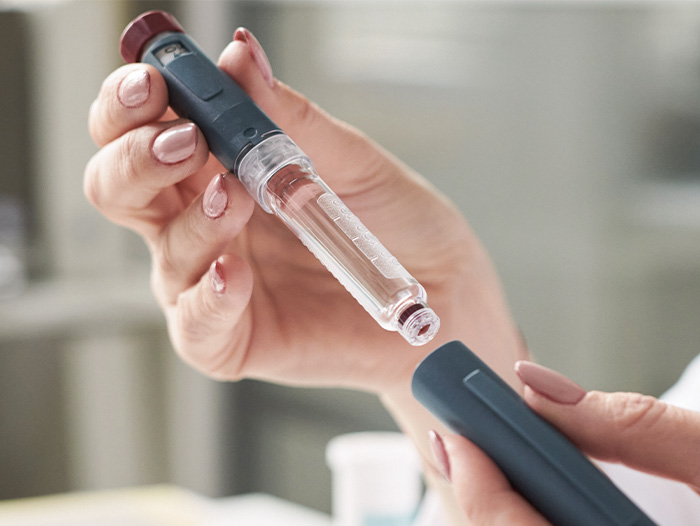
- New Jersey: Novo Nordisk will cut 9,000 jobs, or roughly 11% of its workforce, as part of ongoing restructuring efforts. The layoffs will save the company $1.26 billion a year by the end of 2026 and are part of what Novo described as a broader transformation aimed at simplifying the organization, speeding up decision-making, and pushing more resources to grow its diabetes and obesity businesses. "It is always difficult to see talented and valued colleagues go, but we are convinced that this is the right thing to do for the long-term success of Novo Nordisk," said Novo CEO Mike Doustdar, who stepped into the position last month. "We need a shift in our mindset and approach so we can be faster and more agile." In a video posted on LinkedIn, Doustdar acknowledged the impact of the layoffs and said that "every krone will be reinvested into growth, advancing R&D, expanding manufacturing, and improving commercial execution and patient access worldwide." (Joseph, STAT+ [subscription required], 9/10)
- New Jersey: Novartis last week announced that it plans to acquire Tourmaline Bio in a deal worth roughly $1.4 billion. Through the deal, Novartis will gain access to pacibekitug, a promising experimental drug being tested in atherosclerotic cardiovascular disease and other heart conditions. In May, mid-stage trial data showed that the drug significantly reduced inflammation related to heart disease. "Inflammation is a major driver of cardiovascular disease, and the team at Tourmaline has made significant progress with this asset," said Novartis CMO Shreeram Aradhye. Separately, Tourmaline CEO Sandeep Kulkarni, said that "Novartis shares our conviction in the critical, but largely unaddressed, role of inflammation in driving cardiovascular diseases and will be an ideal partner to accelerate the development of pacibekitug." According to STAT, the acquisition is expected to close by the end of the year. (Joseph, STAT+ [subscription required], 9/9)
- Maryland: FDA has approved a new drug delivery system developed by Johnson & Johnson (J&J) to help treat a type of bladder cancer. The drug release system, which is called Inlexzo, was approved for patients with a type of high-risk non-muscle invasive bladder cancer who did not respond to current standard treatment and are ineligible for or do not wish to undergo bladder removal surgery. Data from a mid-stage study showed that over 82% of patients who received Inlexzo showed no signs of cancer, and over half were cancer-free for at least a year. Inlexzo is inserted directly into the bladder and provides sustained release of the chemotherapy drug gemcitabine for three weeks per treatment cycle for up to 14 cycles. "This drug, at ultra low doses for long periods of time... behaves in a way that not only pushes the disease into remission, but then maintains it through some immune memory," said Christopher Cutie, VP and disease area leader for bladder cancer at J&J. Currently, the company is testing Inlexzo in patients with muscle-invasive bladder cancer to potentially expand its use. (Sunny, Reuters, 9/10)
Our experts weigh in on the state of obesity care, including how far bariatric surgery volumes have dropped, updates on GLP-1 coverage, the rise of new competitors, and what the end of shortages means for compounding.
Don't miss out on the latest Advisory Board insights
Create your free account to access 1 resource, including the latest research and webinars.
Want access without creating an account?
You have 1 free members-only resource remaining this month.
1 free members-only resources remaining
1 free members-only resources remaining
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
This content is available through your Curated Research partnership with Advisory Board. Click on ‘view this resource’ to read the full piece
Email ask@advisory.com to learn more
Click on ‘Become a Member’ to learn about the benefits of a Full-Access partnership with Advisory Board
Never miss out on the latest innovative health care content tailored to you.
Benefits Include:
This is for members only. Learn more.
Click on ‘Become a Member’ to learn about the benefits of a Full-Access partnership with Advisory Board
Never miss out on the latest innovative health care content tailored to you.