Auto logout in seconds.
Continue LogoutCMS is planning to test a new pilot program that would add prior authorization requirements to certain procedures in traditional Medicare, in today's bite-sized hospital and health industry news from Alabama, Georgia, and Maryland.
- Alabama: Late last month, Chief Judge R. David Proctor from Alabama's northern district court approved a $2.8 billion final settlement from the Blue Cross and Blue Shield Association (BCBSA) in litigation that has lasted over 12 years. In 2012, providers sued BCBSA and its 33 member plans, alleging that they colluded for years to decrease reimbursement. Specifically, providers said that the plans' use of "exclusive service areas" created a monopoly that allowed local BCBSA plans to drive up prices. According to Healthcare Dive, settlement discussions began in 2015 and continued for almost a decade before a preliminary settlement was adopted late last year. The $2.8 billion settlement, which plaintiffs' lawyers say is the largest of its kind in the healthcare industry, will be distributed to over 3.3 million providers after attorney's fees and other expenses are taken out. Overall, $1.8 billion has been allocated for healthcare facilities and $152 million has been allocated for medical professionals. However, despite the settlement, litigation against BSBCA continues. In March, dozens of providers filed new suits against BCBSA and its members, seeking greater relief than provided in the current settlement. (Pifer, Healthcare Dive, 8/22)
- Georgia/Maryland: CDC, in coordination with the Maryland Department of Health (MDH), has confirmed the first human case of the New World screwworm in the United States. The New World screwworm is a flesh-eating parasite found in South America and the Caribbean and poses a large risk to livestock. On Aug. 4, CDC and MDH confirmed a human case of the screwworm in a patient who had recently returned from El Salvador. The patient has since recovered from the infection. "This is the first human case of travel-associated New World screwworm myiasis (parasitic infestation of fly larvae) from an outbreak-affected country identified in the United States," said HHS spokesperson Andrew Nixon. "Currently, the risk to public health in the United States from this introduction is very low." Over the last year, the screwworm has been detected in cattle farms in Mexico, which led the United States to temporarily halt live cattle imports from the country. Although the ban was lifted in February, the U.S. Department of Agriculture (USDA) in May reinstated it on a month-by-month basis. USDA has also announced new plans to combat the spread of the screwworm, including build a U.S.-only sterile-fly production facility at an Air Force base in Texas. (Treisman, NPR, 8/25)
- Maryland: CMS is planning to test a new pilot program that would add prior authorization requirements to certain procedures in traditional Medicare. The program, which is called the Wasteful and Inappropriate Service Reduction Model, will examine any practices that are particularly expensive or potentially harmful to patients. It is scheduled to start in six states, Arizona, New Jersey, Ohio, Oklahoma, Texas, and Washington, next year. Currently, the federal government is planning to hire private companies to use AI screening tools to determine whether patients could be covered for certain procedures, such as some spine surgeries or steroid injections. According to the New York Times, the companies selected for the program would have a strong financial incentive to deny claims since they will receive a portion of the savings generated from rejections. Vinay Rathi, a surgeon and expert in Medicare payment policy, said the program could lead to the same problems as Medicare Advantage, where people enroll in private plans. "It's basically the same set of financial incentives that has created issues in Medicare Advantage and drawn so much scrutiny," he said. "It directly puts them at odds with the clinicians." (Abelson/Rosenbluth, New York Times, 8/28)
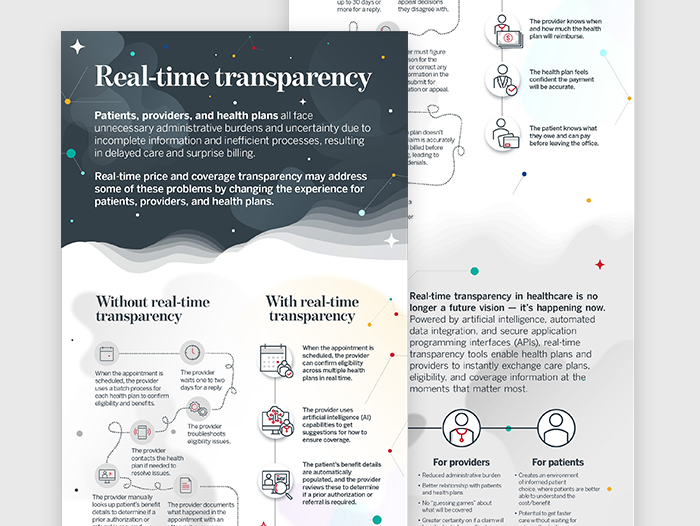
Prior authorizations (PAs) are notorious for their complexity and delays, but GuideWell found a solution. By integrating artificial intelligence (AI) into their PA process, they reduced cycle time to an average of 11 hours and achieved 78% automated approvals within 90 seconds. Discover how GuideWell's innovative approach improved efficiency and member satisfaction.
Don't miss out on the latest Advisory Board insights
Create your free account to access 1 resource, including the latest research and webinars.
Want access without creating an account?
You have 1 free members-only resource remaining this month.
1 free members-only resources remaining
1 free members-only resources remaining
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
You've reached your limit of free insights
Become a member to access all of Advisory Board's resources, events, and experts
Never miss out on the latest innovative health care content tailored to you.
Benefits include:
This content is available through your Curated Research partnership with Advisory Board. Click on ‘view this resource’ to read the full piece
Email ask@advisory.com to learn more
Click on ‘Become a Member’ to learn about the benefits of a Full-Access partnership with Advisory Board
Never miss out on the latest innovative health care content tailored to you.
Benefits Include:
This is for members only. Learn more.
Click on ‘Become a Member’ to learn about the benefits of a Full-Access partnership with Advisory Board
Never miss out on the latest innovative health care content tailored to you.